2019 - Q6
The diagram represents the titration curve for a reaction between a particular acid an particular base.
Which of the following equations best represents the reaction described by the titration curve?
A. NH3(aq) + HCl(aq) --> NH4Cl(aq)
B. NaOH(aq) + HCl(aq) --> NaCl(aq) + H2O(l)
C. NH3(aq) + CH3COOH(aq) --> CH3COONH4(aq)
D. NaOH(aq) + CH3COOH(aq) --> CH3COONa(aq) + H2O(l)

2020 - Q8
A weak base is titrated with 1.0 mol L−1 aqueous HCl. The pH curve is shown.
At which pH value would the solution be most effective as a buffer?
A. 5
B. 7
C. 8
D. 9

2019 - Q22 (4 marks)
A buffer was prepared with acetic acid and sodium acetate. A few drops of universal indicator were then added. When small amounts of either 0.1 mol L–1 HCl(aq) or 0.1 mol L–1 NaOH(aq) were added, no change in the colour of the solution was observed.
Explain these observations. Support your answer with at least ONE chemical equation.
2019 - Q24 (7 marks)
A conductometric titration was undertaken to determine the concentration of a barium hydroxide solution. The solution was added to 250.0 mL of standardised 1.050 × 10–3 mol L–1 hydrochloric acid solution. The results of the titration are shown in the conductivity graph.
(a) Explain the shape of the titration curve. (3 marks)
(b) The equivalence point was reached when a volume of 17.15 mL of barium hydroxide was added.
Calculate the concentration of barium hydroxide (in mol L–1), and give a relevant chemical equation. (4 marks)

2019 - Q27 (5 marks)
The relationship between the acid dissociation constant, Ka , and the corresponding conjugate base dissociation constant, Kb , is given by:
Ka × Kb = Kw
Assume that the temperature for part (a) and part (b) is 25°C.
(a) The Ka of hypochlorous acid (HOCl) is 3.0 × 10−8. Show that the Kb of the hypochlorite ion, OCl– , is 3.3 × 10−7. (1 mark)
(b) The conjugate base dissociation constant, Kb , is the equilibrium constant for the following equation:
Calculate the pH of a 0.20 mol L−1 solution of sodium hypochlorite (NaOCl). (4 marks)
2021 - Q5
A student used the following method to titrate an acetic acid solution of unknown concentration with a standardised solution of dilute sodium hydroxide.
• Rinse burette with deionised water.
• Fill burette with sodium hydroxide solution.
• Rinse pipette and conical flask with acetic acid solution.
• Pipette 25.00 mL of acetic acid solution into conical flask.
• Add appropriate indicator to the conical flask.
• Titrate to endpoint and record volume of sodium hydroxide solution used.
Compared to the actual concentration of the acetic acid, the calculated concentration will be
A. lower.
B. higher.
C. the same.
D. different, but higher or lower cannot be predicted.
2021 - Q6
Which row of the table describes what happens when a solution of a weak acid is diluted? (Assume constant temperature.)

2021 - Q16
This titration curve is produced when an acid is titrated with a sodium hydroxide solution of the same concentration.
How many acidic protons does this acid possess?
A. 1
B. 2
C. 3
D. 4

2022 - Q2
When a solution of a primary standard is prepared for titration, which of the following is required?
A. A burette
B. A balance
C. An indicator
D. A condenser
2020 - Q28 ( 3 marks)
A chemist used the following method to determine the concentration of a dilute solution of propanoic acid (pKa = 4.88).
The chemist weighed out 1.000 g of solid NaOH on an electronic balance and then made up the solution in a 250.0 mL volumetric flask.
The chemist then performed titrations, using bromocresol green as the indicator. This indicator is yellow below pH 3.2 and green above pH 5.2 .
The results are shown in the table.
Explain why this method produces inaccurate and unreliable results.

2021 - Q35 (7 marks)
A manufacturer requires that its product contains at least 85% v/v ethanol.
The concentration of ethanol in water can be determined by a back titration. Ethanol is first oxidised to ethanoic acid using an excess of acidified potassium dichromate solution.
Does the sample meet the manufacturer’s requirements? Support your answer with calculations.

2021 - Q36 (5 marks)

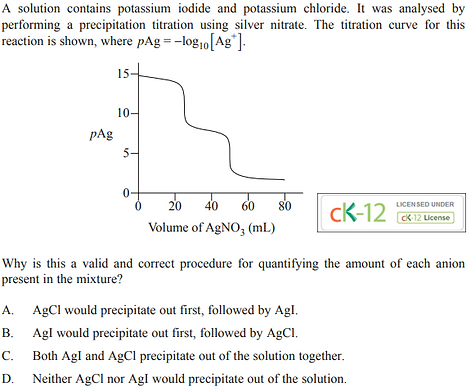
2022 - Q15

2022 - Q20

2022 - Q32 (8 marks)
The concentration of citric acid, a triprotic acid, in a carbonated soft drink was to be determined.
Step 1: A solution of NaOH(aq) was standardised by titrating it against 25.00 mL aliquots of a solution of the monoprotic acid potassium hydrogen phthalate (KHP). The KHP solution was produced by dissolving 4.989 g in enough water to make 100.0 mL of solution. The molar mass of KHP is 204.22 g mol−1.
The results of the standardisation titration are given in the table.
Step 2: A 75.00 mL bottle of the drink was opened and the contents quantitatively transferred to a beaker. The soft drink was gently heated to remove CO2.
Step 3: The cooled drink was quantitatively transferred to a 250.0 mL volumetric flask and distilled water was added up to the mark.
Step 4: 25.00 mL samples of the solution were titrated with the NaOH(aq) solution. The average volume of NaOH(aq) used was 13.10 mL.
(a) Calculate the concentration of the triprotic citric acid in the soft drink. (6 marks)
(b) Explain how your answer to part (a) would be different if the carbon dioxide was not removed from the soft drink. (2 marks)

2023 - Q23 (3 marks)

2023 - Q8
2023 - Q35 (6 marks)



2023 - Q14

2023 - Q14