2019 - Q7
How does the addition of a catalyst affect a reversible reaction?
A. It increases the activation energy of the forward reaction only.
B. It decreases the activation energy of the forward reaction only.
C. It increases the activation energy of both the forward and reverse reactions.
D. It decreases the activation energy of both the forward and reverse reactions.
2019 - Q12
Methanol can be produced from the reaction of carbon monoxide and hydrogen, according to the following equation:
Which set of conditions will produce the maximum yield of methanol?
A. Low pressure and low temperature
B. Low pressure and high temperature
C. High pressure and low temperature
D. High pressure and high temperature
2020 - Q16
Compounds X, Y and Z are in equilibrium. The diagram shows the effects of temperature and pressure on the equilibrium yield of compound Z.

(a) The concentrations of reactants and products as a function of time for the following system were determined.
At time T, some CO(g) was removed from the system.
The concentration of CO after time T is shown. Sketch the concentrations after time T for the remaining species. (2 marks)
(b) Using collision theory, explain the change in the concentration of CO after time T. (3 marks)

2021 - Q22 (3 marks)
Consider the following equilibrium system.
The solution is orange.
Justify TWO ways to shift the equilibrium to the left to change the colour of the solution.

2020 - Q19
Nitrogen dioxide reacts to form dinitrogen tetroxide in a sealed flask according to the following equation.
Which graph best represents the rates of both the forward and reverse reactions when an equilibrium system containing these gases is cooled at time t ?
2022 - Q23 (6 marks)
Consider the following system which is at equilibrium in a rigid, sealed container.
4NH3(g) + 5O2(g) <--> 4NO(g) + 6H2O(g) deltaH = −950 kJ mol−1
(a) Identify what would happen to the amount of NO(g) if the temperature was increased. (1 mark)
(b) Explain why a catalyst does not affect the equilibrium position of this system. (2 marks)
(c) Using collision theory, explain what would happen to the concentration of NO(g) if H2O(g) was removed from the system. (3 marks)

2023 - Q26 (2 of 5 marks)
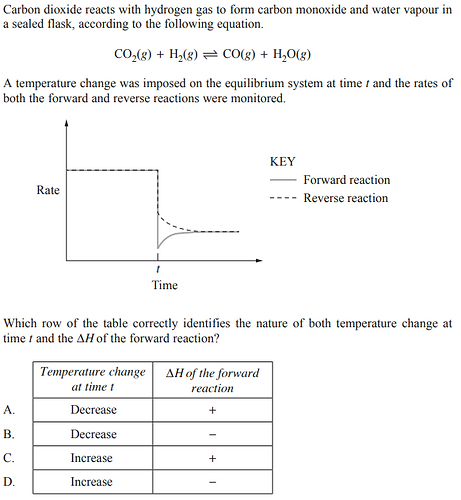
2020 - Q19
Nitrogen dioxide reacts to form dinitrogen tetroxide in a sealed flask according to the following equation.
Which graph best represents the rates of both the forward and reverse reactions when an equilibrium system containing these gases is cooled at time t ?


2023 - Q33 (6 marks)
2021 - Q11
Consider this system in a fixed volume at constant temperature.
PCl5(s) <--> PCl3(l) + Cl2(g)
This system is initially at equilibrium. A small amount of solid PCl5 is added.
Which statement is correct?
A. The amount of Cl2 will increase.
B. The amount of PCl3 will decrease.
C. The amount of Cl2 will not change.
D. The amount of PCl5 will increase then decrease.

2023 - Q12


2023 - Q18