2019 - Q16
At equilibrium, a 1.00 L vessel contains 0.0430 mol of H2, 0.0620 mol of I2, and 0.358 mol of HI.
The system is represented by the following equation:
H2(g) + I2(g) <--> 2HI(g)
Which of the following is closest to the value of the equilibrium constant,
Keq, for this reaction?
A. 0.0208
B. 48.1
C. 134
D. 269
2020 - Q14
The equation for the autoionisation of water is shown.
2H2O(l) <--> H3O+(aq) + OH−(aq)
At 50°C the water ionisation constant, Kw , is 5.5 × 10^−14.
What is the pH of water at 50°C?
A. 5.50
B. 6.63
C. 6.93
D. 7.00
2022 - Q8

2022 - Q13
Nitrosyl bromide decomposes according to the following equation.
A 0.64 mol sample of NOBr is placed in an evacuated 1.00 L flask.
After the system comes to equilibrium, the flask contains 0.46 mol NOBr.
What are the concentrations of NO and Br2 in the flask at equilibrium?

2019 - Q31 (4 marks)
The following reaction occurs in an aqueous solution.

2020 - Q27 (5 marks)
A student makes up a solution of propan-2-amine in water with a concentration of 1.00 mol L–1.
(b) The equilibrium constant for the reaction of propan-2-amine with water is 4.37 × 10−4. Calculate the concentration of hydroxide ions in this solution. (3 marks)
2020 - Q35 (4 marks)

Determine the value of the equilibrium constant, given that A = 0.745 at equilibrium and [ I− ]initial = 7.00 × 10−4 mol L−1.
2021 - Q31 (4 marks)
Ammonia is produced according to the following equilibrium equation.
N2(g) + 3H2(g) <--> 2NH3 (g)
There are 4.50 moles of nitrogen gas, 1.00 mole of hydrogen gas and 5.80 moles of ammonia in a 10.0 L vessel. The system is at equilibrium at 298 K. The value of Keq at this temperature is 748.
How many moles of nitrogen gas need to be added to the vessel to increase the amount of ammonia by 0.050 moles?
2022 - Q31 (7 marks)
Silver ions form the following complex with ammonia solution.
The equilibrium constant is 1.6 × 10^7 at 25°C
(a) In order to determine the free Ag+ concentration in an aqueous ammonia solution, a student carried out a precipitation titration with NaI(aq) as the titrant.
Evaluate the suitability of this method. (3 marks)
(b) If 0.010% of the total silver ions in solution are present as Ag+(aq) at equilibrium, calculate the equilibrium concentration of aqueous ammonia in this solution. (4 marks)
2023 - Q31 (7 marks)
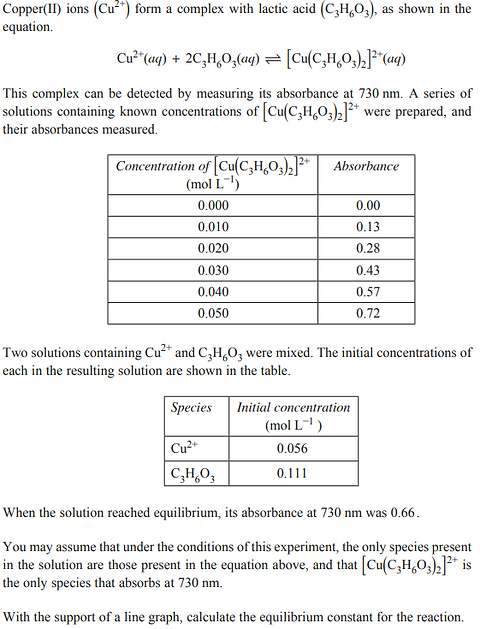
2022 - Q14
Nitrogen dioxide can react with itself to produce dinitrogen tetroxide.
2NO2(g) <--> N2O4(g) Keq = 0.010
In an experiment, 100.0 cm3 of NO2 is placed in a syringe. The plunger is then pushed in until the volume is 50.0 cm3 , while maintaining a constant temperature.
The system is allowed to return to equilibrium.
Which statement is true for the system at equilibrium?

2023 - Q7

2023 - Q20

2023 - Q37 (5 marks)
