Barker College Chemistry Department
Past HSC Chemistry Questions
Please click the following link for the past papers of the new syllabus: HOME | Past Hsc Chem3 (andrewchoi2.wixsite.com)
2002 - Q6
Which is amphiprotic?

2002 - Q7
What did the Bronsted-Lowry definition of acids identify that made it a significant improvement over earlier definitions?
(A) Acids contain hydrogen
(B) Acids are proton donors
(C) Acids contain oxygen
(D) Acids are electron-pair acceptors
2002 - Q8
In a titration, an acid of known concentration is placed in a burette and reacted with a base that has been pipetted into a conical flask.
What should each piece of glassware be rinsed with immediately before the titration?
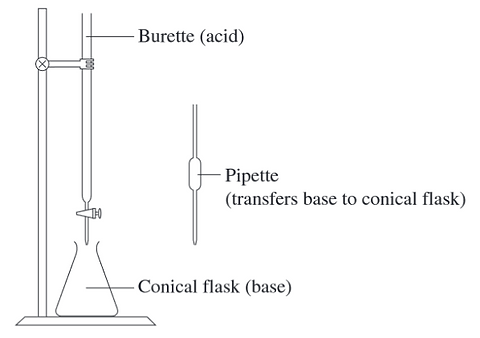

2003 - Q14
In a titration of a strong base with a strong acid, the following procedure was used:
1. A burette was rinsed with water and then filled with the standard acid.
2. A pipette was rinsed with some base solution.
3. A conical flask was rinsed with some base solution.
4. A pipette was used to transfer a measured volume of base solution into the conical flask.
5. Indicator was added to the base sample and it was titrated to the endpoint with the acid.
Which statement is correct?
(A) The calculated base concentration will be correct.
(B) The calculated base concentration will be too low.
(C) The calculated base concentration will be too high.
(D) No definite conclusion can be reached about the base concentration.
2001 - Q21 (4 marks)
Barium hydroxide and sulfuric acid react according to the following equation:
Ba(OH)2 (aq) + H2SO4 (aq) ---> BaSO4 (s) + 2H2O (l)
(a) Name this type of chemical reaction. (1 mark)
(b) A 20mL sample of barium hydroxide was titrated with 0.12 M sulfuric acid. The conductivity of the solution was measured throughout the titration and the results graphed, as shown.
Explain the changes in conductivity shown by the graph. (3 marks)

2001 - Q23 (4 marks)
A household cleaning agent contains a weak base of a general formula NaX. 1.00 g of this compound was dissolved in 100.0 mL of water. A 20.0 mL sample of the solution was titrated with 0.1000 M hydrochloric acid and required 24.4 mL of the acid for neutralisation.
(a) What is the Bronsted-Lowry definition of a base? (1 mark)
(b) What is the molar mass of the base? (3 marks)
2003 - Q23 (4 marks)
25.0 mL of 0.12 M standard barium hydroxide solution was titrated with nitric acid. The results are recorded in the table.
(a) Write a balanced chemical equation for the reaction of barium hydroxide with nitric acid. (1 mark)
(b) Calculate the concentration of the nitric acid. (3 marks)

2003 - Q24 (4 marks)
Discuss factors that must be considered when using neutralisation reactions to safely minimise damage in chemical spills.
2004 - Q16 (5 marks)
(a) Outline the procedure you would use to prepare a standard solution of sodium hydrogen carbonate from solid sodium hydrogen carbonate. (3 marks)
(b) Calculate the mass of solid sodium hydrogen carbonate required to make 250 mL of 0.12 mol L−1 solution. (2 marks)
2003 - Q15
Which of the following graphs shows how pH will vary when dilute HCl is added to 100 mL of dilute natural buffer solution with an initial pH of 7.0?

2004 - Q5
Which statement best represents Davy’s definition of an acid?
(A) Acids contain oxygen.
(B) Acids are proton donors.
(C) Acids contain replaceable hydrogen.
(D) Acids ionise in solution to form hydrogen ions
2005 - Q9
Which of the following pairs would form a buffer solution?
(A) HCl(aq) / Cl− (aq)
(B) H2PO4 − (aq) / PO4 3−(aq)
(C) H2SO4(aq) / HSO4 − (aq)
(D) CH3COOH(aq) / CH3COO− (aq)
2005 - Q10
A titration was conducted by adding NaOH from a teflon-coated burette to HCl in a conical flask. The pH in the flask was recorded during the titration and Curve A was produced.


2004 - Q22 (3 marks)
(a) Define the term amphiprotic. (1 mark)
(b) Write TWO chemical equations to show that the dihydrogen phosphate ion (H2PO4 − ) is amphiprotic. (2 marks)
2005 - Q21 (5 marks)
Analyse how knowledge of the composition and properties of acids has led to changes in the definition of acids.
2005 - Q24 (5 marks)
An antacid tablet is known to contain calcium carbonate (CaCO3). To determine the mass of calcium carbonate in the tablet, the following procedure was used.
• The tablet was crushed and then placed in a beaker.
• A pipette was used to add 25.0 mL of 0.600 mol L–1 hydrochloric acid to the crushed tablet in the beaker.
• Once the reaction between the calcium carbonate and hydrochloric acid had stopped, phenolphthalein indicator was added to the reaction mixture.
• A teflon-coated burette was then used to add 0.100 mol L–1 sodium hydroxide to the beaker to neutralise the excess hydrochloric acid.
• The phenolphthalein changed from colourless to pink after 14.2 mL of the sodium hydroxide solution had been added.
(a) Write a balanced chemical equation for the reaction that occurred between the calcium carbonate in the tablet and the hydrochloric acid. (1 mark)
(b) How many moles of hydrochloric acid were added to the tablet? (1 mark)
(c) Calculate the mass of calcium carbonate in the original antacid tablet. (3 marks)
2006 - Q21 (3 marks)
You performed a first-hand investigation to identify the pH of a range of salt solutions.
(a) Identify an acidic salt you used. (1 mark)
(b) Explain the acidic nature of the salt you selected. Include a balanced chemical equation in your answer (2 marks).
2006 - Q27 (4 marks)
One of the most common methods for determining the concentration of metal ions in water samples involves titration with a reagent called EDTA. In alkaline solution EDTA is present as an anion with a 4 – charge. In this form it reacts with metal ions such as calcium and magnesium in a 1 : 1 ratio:
Ca 2+ + EDTA 4– → Ca(EDTA) 2–
When the reaction between the metal ions and EDTA 4– is complete, an indicator also present in the solution changes colour.
A student used the following procedure to determine the concentration of calcium in a sample of water:
• 50.0 mL of water sample was pipetted into a conical flask
• 5.0 mL of ammonia/ammonium ion buffer and two drops of indicator were added
• Sample was titrated with 0.0200 mol L–1 EDTA 4– until indicator changed colour • The above procedure was repeated a further three times
• The average volume of EDTA 4– used in the four titrations was 24.0 mL
(a) What is the average number of moles of EDTA 4– added to reach the end point? (1 mark)
(b) The student used the answer to part (a) to calculate the concentration of Ca 2+ in the water sample in mg L–1. What concentration was obtained? (2 marks)
(c) The concentration of Ca 2+ in the water sample was also determined by atomic absorption spectroscopy, and found to be 16% lower than the value obtained by titration with EDTA 4–.
Suggest a reason why the concentration of Ca 2+ determined by EDTA titration was higher. (1 mark)
2007 - Q25 (5 marks)
Sodium hydrogen carbonate, NaHCO3, is commonly used to neutralise chemical spills that are a potential hazard to the environment.
Assess the effectiveness of NaHCO3 in this role, with reference to its chemical properties.
2008 - Q26 (4 marks)
Explain how a buffer works with reference to a specific example in a natural system.
2006 - Q9
Which statement best describes the equivalence point in a titration between a strong acid and a strong base?
(A) The point at which the first sign of a colour change occurs
(B) The point at which equal moles of acid and base have been added together
(C) The point at which equal moles of H+ ions and OH– ions have been added together
(D) The point at which the rate of the forward reaction equals the rate of the reverse reaction
2008 - Q27 (4 marks)
(a) Classify these salts as forming acidic, basic or neutral solutions. (2 marks)
Salt Classification of solution
Ammonium chloride
Sodium ethanoate
Sodium chloride
Ammonium nitrate
(b) From the table, choose a salt that forms an acidic or basic solution, and justify its classification. Include an equation to illustrate your answer. (2 marks)
2006 - Q11
In 1884, Svante Arrhenius proposed a definition for acids. His definition was soon accepted as superior to that put forward by earlier chemists.
Why was Arrhenius’ definition seen as a major improvement?
(A) It explained why some acids do not contain oxygen.
(B) It showed how the solvent can affect the strength of an acid.
(C) It showed the relationship between pH and the concentration of H+ ions.
(D) It could be used to explain why some acids are strong and others are weak.
2007 - Q7
Which graph represents the enthalpy change for an acid-base neutralisation reaction?

2007 - Q9
Which of the following aqueous solutions has a pH greater than 7?
(A) Sodium citrate
(B) Sodium chloride
(C) Ammonium nitrate
(D) Ammonium chloride
2008 - Q8
According to the Arrhenius theory of acids and bases, an acid is a substance that
(A) tastes sour.
(B) is capable of donating a hydrogen ion.
(C) can accept a pair of electrons to form a co-ordinate covalent bond.
(D) increases the concentration of hydrogen ions in an aqueous solution.
2009 - Q7
What is the conjugate base of HSO4 – ?
(A) SO3 2–
(B) SO4 2–
(C) H2SO4
(D) HSO –
2009 - Q14
Citric acid, the predominant acid in lemon juice, is a triprotic acid. A student titrated 25.0 mL samples of lemon juice with 0.550 mol L–1 NaOH. The mean titration volume was 29.50 mL. The molar mass of citric acid is 192.12 g mol–1.
What was the concentration of citric acid in the lemon juice?
(A) 1.04 g L–1
(B) 41.6 g L–1
(C) 125 g L–1
(D) 374 g L–1
2010 - Q8
In a research report a student wrote, ‘Acids are compounds that contain hydrogen and can dissolve in water to release hydrogen ions into solution.’ Who originally stated this theory of acids?
(A) Arrhenius
(B) Brönsted–Lowry
(C) Davy
(D) Lavoisier
2011 - Q15 - 16
Using 0.100 mol L–1 NaOH, a student titrated 25.0 mL of a 0.100 mol L–1 weak monoprotic acid, and separately titrated 25.0 mL of a 0.100 mol L–1 strong monoprotic acid.
(15) Which statement about the volume of base required to reach the equivalence point is correct?
(A) The weak acid will require the same volume of base as the strong acid. (B) The weak acid will require a larger volume of base than the strong acid. (C) The weak acid will require a smaller volume of base than the strong acid.
(D) The volume of base required will depend on the molar mass of the acid used.
(16) Which statement correctly describes the pH at each titration equivalence point?
(A) The pH of both solutions will be the same.
(B) One of the solutions will be neutral while the other will have a pH higher than 7.
(C) One of the solutions will be neutral while the other will have a pH lower than 7.
(D) One of the solutions will have a pH higher than 7 while the other will have a pH lower than 7.
2011 - Q18
A household cleaning agent contains a weak base with the formula NaX. 1.00 g of this compound was dissolved in water to give 100.0 mL of solution. A 20.0 mL sample of the solution was titrated with 0.100 mol L–1 hydrochloric acid, and required 24.4 mL of the acid for neutralisation.
What is the molar mass of the weak base?
(A) 82.0 g
(B) 84.0 g
(C) 122 g
(D) 410 g
2008 - Q28 (6 marks)
A standard solution was prepared by dissolving 1.314 g of sodium carbonate in water. The solution was made up to a final volume of 250.0 mL.
(a) Calculate the concentration of the sodium carbonate solution. (2 marks)
This solution was used to determine the concentration of a solution of hydrochloric acid. Four 25.00 mL samples of the acid were titrated with the sodium carbonate solution. The average titration volume required to reach the end point was 23.45 mL.
(b) Write a balanced equation for the titration reaction. (1 mark)
(c) Calculate the concentration of the hydrochloric acid solution. (2 marks)
2009 - Q21 (6 marks)
The graph shows changes in pH for the titrations of equal volumes of solutions of two monoprotic acids, Acid 1 and Acid 2.
(a) Explain the differences between Acid 1 and Acid 2 in terms of their relative strengths and concentrations. (3 marks)
(b) Name the salt produced by the reaction of an acid of the same type as Acid 2 with KOH(aq). (1 mark)
(c) Calculate the concentration of hydrogen ions when 20 mL of KOH(aq) has been added to Acid 1. (1 mark)
(d) Why would phenolphthalein be a suitable indicator for both titrations? (1 mark)

2009 - Q22 (7 marks)
The nitrogen content of bread was determined using the following procedure:
• A sample of bread weighing 2.80 g was analysed.
• The nitrogen in the sample was converted into ammonia.
• The ammonia was collected in 50.0 mL of 0.125 mol L−1 hydrochloric acid. All of the ammonia was neutralised, leaving an excess of hydrochloric acid.
• The excess hydrochloric acid was titrated with 23.30 mL of 0.116 mol L−1 sodium hydroxide solution.
(a) Write balanced equations for the TWO reactions involving hydrochloric acid. (2 marks)
(b) Calculate the moles of excess hydrochloric acid. (1 mark)
(c) Calculate the moles of ammonia. (2 marks)
(d) Calculate the percentage by mass of nitrogen in the bread. (2 marks)
2010 - Q28 (8 marks)
The flowchart shown outlines the sequence of steps used to determine the concentration of an unknown hydrochloric acid solution.
Describe steps A, B and C including correct techniques, equipment and appropriate calculations. Determine the concentration of the hydrochloric acid.

2011 - Q25 (3 marks)
Explain the role of the conjugate acid/base pair, H2PO4 - / HPO4 2– , in maintaining the pH of living cells. Include chemical equations in your answer.
2011 - Q26 (6 marks)
A manufacturer makes lemon cordial by mixing flavouring, sugar syrup and citric acid. The concentration of the citric acid is determined by titration with NaOH.
The sodium hydroxide solution is prepared by dissolving 4.000 g of NaOH pellets in water to give 1.000 L of solution. This solution is standardised by titrating 25.00 mL with a 0.1011 mol L–1 standardised solution of HCl. The average titration volume is found to be 24.10 mL.
To analyse the lemon cordial 50.00 mL of the cordial is diluted to 500.0 mL. Then 25.00 mL of the diluted solution is titrated with the NaOH solution to the phenolphthalein endpoint.
The following data were collected during one of the analysis runs of the lemon cordial.
Titration #1 volume 26.55 mL
Titration #2 volume 27.25 mL
Titration #3 volume 27.30 mL
Titration #4 volume 27.20 mL
(a) Why is the calculated concentration of the standardised NaOH solution different from the concentration calculated using the mass given, assuming no human error occurred? (2 marks)
(b) Determine the concentration of citric acid in the lemon cordial. (4 marks)
2012 - Q4
Which pieces of glassware should be used when preparing a primary standard solution?
(A) Pipette, burette and conical flask
(B) Dropper, watch glass and pipette
(C) Beaker, filter funnel and volumetric flask
(D) Measuring cylinder, stirring rod and conical flask
2011 - Q29 (4 marks)
(a) Justify the continued use of the Arrhenius definition of acids and bases, despite the development of the more sophisticated Brönsted–Lowry definition. (3 marks)
(b) Why does the neutralisation of any strong acid in an aqueous solution by any strong base always result in a heat of reaction of approximately –57 kJ mol −1 ? (1 mark)
2012 - Q8
Which acid / base pair could act as a buffer?
(A) H3O + / H2O
(B) H2O / OH –
(C) HNO3 / NO3 –
(D) H2PO4 - / HPO4 2-
2013 - Q17
A 25.0 mL sample of a 0.100 mol L–1 hydrochloric acid solution completely reacted with 23.4 mL of sodium hydroxide solution. What volume of the same sodium hydroxide solution would be required to completely react with 25.0 mL of a 0.100 mol L–1 acetic acid solution?
(A) Less than 23.4 mL
(B) 23.4 mL
(C) More than 23.4 mL
(D) Unable to calculate unless the concentration of the sodium hydroxide solution is also known
2014 - Q3
Which row of the table correctly matches the scientist(s) with their theory of acids?
Scientist(s) Theory
(A) Arrhenius Acids contain oxygen
(B) Brönsted and Lowry Acids are proton donors
(C) Davy Acids are able to produce hydrogen ions in water
(D) Lavoisier Acids contain hydrogen
2014 - Q10
The following equation represents a chemical system in equilibrium:
OCl− (aq) + H2O(l) <----> HOCl(aq) + OH− (aq)
Which of the following is an acid/base conjugate pair?
(A) H2O / HOCl
(B) HOCl / OH−
(C) HOCl / OCl−
(D) OCl− / H2O
2015 - Q2
Which type of glassware is used in a titration to deliver an accurate volume of a solution to a known volume of another solution?

2015 - Q14
The graph shows the changes in pH during a titration.
Which pH range should an indicator have to be used in this titration?
(A) 3.1– 4.4
(B) 5.0 – 8.0
(C) 6.0 – 7.6
(D) 8.3 –10.0

2016 - Q10
Which of the following is the conjugate base of the H2PO4 1– ion?
(A) H3PO4
(B) H3PO3
(C) HPO4 2–
(D) HPO3 2–
2017 - Q1
In an experiment, 30 mL of water is to be transferred into a conical flask.
Which piece of equipment would deliver the volume with the greatest accuracy?
(A) Burette
(B) Beaker
(C) Test tube
(D) Measuring cylinder
2017 - Q5
Which of the following substances is amphiprotic in nature?
A. HSO4 –
B. H2SO4
C. SO4 2–
D. H2SO3
2012 - Q30 (6 marks)
A chemist analysed aspirin tablets for quality control. The initial step of the analysis was the standardisation of a NaOH solution. Three 25.00 mL samples of a 0.1034 mol L–1 solution of standardised HCl were titrated with the NaOH solution. The average volume required for neutralisation was 25.75 mL.
(a) Calculate the molarity of the NaOH solution. (2 marks)
Three flasks were prepared each containing a mixture of 25 mL of water and 10 mL of ethanol. An aspirin tablet was dissolved in each flask. The aspirin in each solution was titrated with the standardised NaOH solution according to the following equation:
C9H8O4(aq) + NaOH(aq) → C9H7O4Na(aq) + H2O(l)
The following titration results were obtained.
Tablet Volume (mL)
1 16.60
2 16.50
3 16.55
(b) (i) Calculate the average mass (mg) of aspirin per tablet. (3 marks)
(ii) Why was it necessary to include the ethanol in the mixture? (1 mark)
2013 - Q28 (5 marks)
A student attempted to determine the concentration of a hydrochloric acid solution. The following steps were performed.
Step 1. A conical flask was rinsed with water.
Step 2. A 25.0 mL pipette was rinsed with water.
Step 3. The student filled the pipette with a standard sodium carbonate solution to the level shown in the diagram.
Step 4. The standard sodium carbonate solution in the pipette was transferred to the conical flask. The student ensured that all of the sodium carbonate solution was transferred to the conical flask by blowing through the pipette. Three drops of an appropriate indicator were added to the conical flask.
Step 5. A burette was rinsed with the hydrochloric acid solution and then filled with the acid. The student then carried out a titration to determine the concentration of the hydrochloric acid solution.
In steps 2, 3 and 4 above the student did not follow acceptable procedures.
(a) Identify the mistake the student made in step 4 and propose a change that would improve the validity of the result. (2 marks)
(b) Explain the effect of the mistakes made in steps 2 and 3 on the calculation of the concentration of the hydrochloric acid solution. (3 marks)

2014 - Q30 (5 marks)
A batch of dry ice (solid CO2 ) was contaminated during manufacture. To determine its purity, the following steps were carried out.
Step 1: A 0.616 gram sample of the contaminated dry ice was placed in a clean, dry flask.
Step 2: 50.00 mL of 1.00 mol L−1 sodium hydroxide was added to the flask. The sodium hydroxide was in excess.
Step 3: The flask was sealed to prevent loss of carbon dioxide gas and the reaction allowed to reach completion, according to this equation:
2NaOH(aq) + CO2(s) → Na2CO3 (aq) + H2O(l)
Step 4: The remaining sodium hydroxide was titrated against a 1.00 mol L−1 solution of hydrochloric acid. The average volume of HCl used was 27.60 mL.
(a) Calculate the number of moles of NaOH added in Step 2. (1 mark)
(b) Calculate the percentage purity by mass of this batch of dry ice. (4 marks)
2015 - Q24 (5 marks)
(a) Explain why the salt, sodium acetate, forms a basic solution when dissolved in water. Include an equation in your answer. (2 marks)
(b) A solution is prepared by using equal volumes and concentrations of acetic acid and sodium acetate.
Explain how the pH of this solution would be affected by the addition of a small amount of sodium hydroxide solution. Include an equation in your answer. (3 marks)
2015 - Q28 (3 marks)
The equipment shown is set up. After some time a ring of white powder is seen to form on the inside of the glass tube.
(a) Why would this NOT be an acid–base reaction according to Arrhenius? (1 mark)
(b) Explain why this would be considered a Brönsted–Lowry acid–base reaction. Include an equation in your answer. (2 marks)

2016 - Q29 (6 marks)
A solution of hydrochloric acid was standardised by titration against a sodium carbonate solution using the following procedure.
• All glassware was rinsed correctly to remove possible contaminants.
• Hydrochloric acid was placed in the burette.
• 25.0 mL of sodium carbonate solution was pipetted into the conical flask.
The titration was performed and the hydrochloric acid was found to be 0.200 mol L–1.
(a) Identify the substance used to rinse the conical flask and justify your answer. (2 marks)
(b) Seashells contain a mixture of carbonate compounds. The standardised hydrochloric acid was used to determine the percentage by mass of carbonate in a seashell using the following procedure.
• A 0.145 g sample of the seashell was placed in a conical flask.
• 50.0 mL of the standardised hydrochloric acid was added to the conical flask.
• At the completion of the reaction, the mixture in the conical flask was titrated with 0.250 mol L–1 sodium hydroxide.
The volume of sodium hydroxide used in the titration was 29.5 mL.
Calculate the percentage by mass of carbonate in the sample of the seashell. (4 marks)